Absolute and relative rate constants for the reactions of OH and Cl with pentanols
Résumé
The rate constants for the reactions of OH with three pentanols have been determined in the range 267−373 K and P = 100 Torr. The data obtained were (in units of cm3molecule−1s−1): k(1-pentanol) = (6.7 ± 3.8) × 10−12 exp [(132 ± 176)/T], k(2-pentanol) = (5.2 ± 1.8) × 10−12 exp [(218 ± 116)/T], k(3-pentanol) = (5.8 ± 2.3) × 10−12 exp [(164 ± 118)/T]. The present work provides the first temperature dependence data on these reactions. In addition, using the relative rate method, the rate constants for OH and Cl with these pentanols have been measured. The results are compared with the literature data and discussed with respect to atmospheric chemistry.
Domaines
Sciences de la Terre
Fichier principal
 Pentanols_OH_Cl_CPL.pdf (210.96 Ko)
Télécharger le fichier
Pentanols_OH_Cl_CPL.pdf (210.96 Ko)
Télécharger le fichier
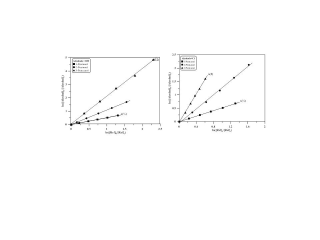 Fig1-Lendar.jpg (22.25 Ko)
Télécharger le fichier
Fig1-Lendar.jpg (22.25 Ko)
Télécharger le fichier
| Origine | Fichiers produits par l'(les) auteur(s) |
|---|
| Format | Figure, Image |
|---|
Loading...
