Structure, Orientation and Stability of Lysozyme Confined in layered Materials
Résumé
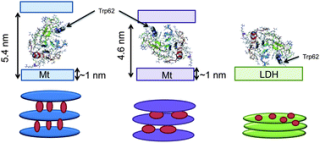
The confinement of lysozyme in 3 layered materials based on montmorillonite and lamellar double hydroxide exhibiting different surface charges was studied. The protein structure and orientation in these materials were determined by X-Ray diffraction, time resolved fluorescence and fluorescence anisotropy. For both Montmorillonite exchanged with sodium and modified with non-ionic surfactant (tri-ethylene glycol mono n-decyl ether), the lysozyme was found to be located in the interlayer space with "end-on" and "side-on" orientation, respectively. Conversely no lysozyme intercalation was observed with lamellar double hydroxide modified with anionic surfactant (sodium octylsulfate), since protein was adsorbed on the surface of the particles. Fourier transformed infra-red spectroscopy analysis shows that lysozyme confinement in the interlayer space preserves its structure after dehydration, whereas some structural changes were observed for lysozyme adsorbed on the particle surface.
Fichier principal
 manuscript-revised.pdf (1012.19 Ko)
Télécharger le fichier
manuscript-revised.pdf (1012.19 Ko)
Télécharger le fichier
 GA.gif (21.03 Ko)
Télécharger le fichier
GA.gif (21.03 Ko)
Télécharger le fichier
| Origine | Fichiers éditeurs autorisés sur une archive ouverte |
|---|
| Format | Figure, Image |
|---|
Loading...
